Molecular Mechanisms of Intracellular Trafficking
Dr. Vytautė Starkuvienė
We aim to uncover how the precise spratial and temporal regulation of intracellular trafficking is achieved.
Intracellular trafficking ensures the distribution of newly synthesized and internalized molecules to their appropriate subcellular sites for action or degradation, thereby maintaining tissue homeostasis. Additionally, we aim to determine how its deregulation contributes to disease and accelerates aging. To address these questions, we employ a broad array of techniques, including high-content microscopy-based assays, 2D and 3D cellular models, RNAi, CRISPR-Cas9 gene editing, and antibody-mediated loss-of-function approaches.
Research Strategy
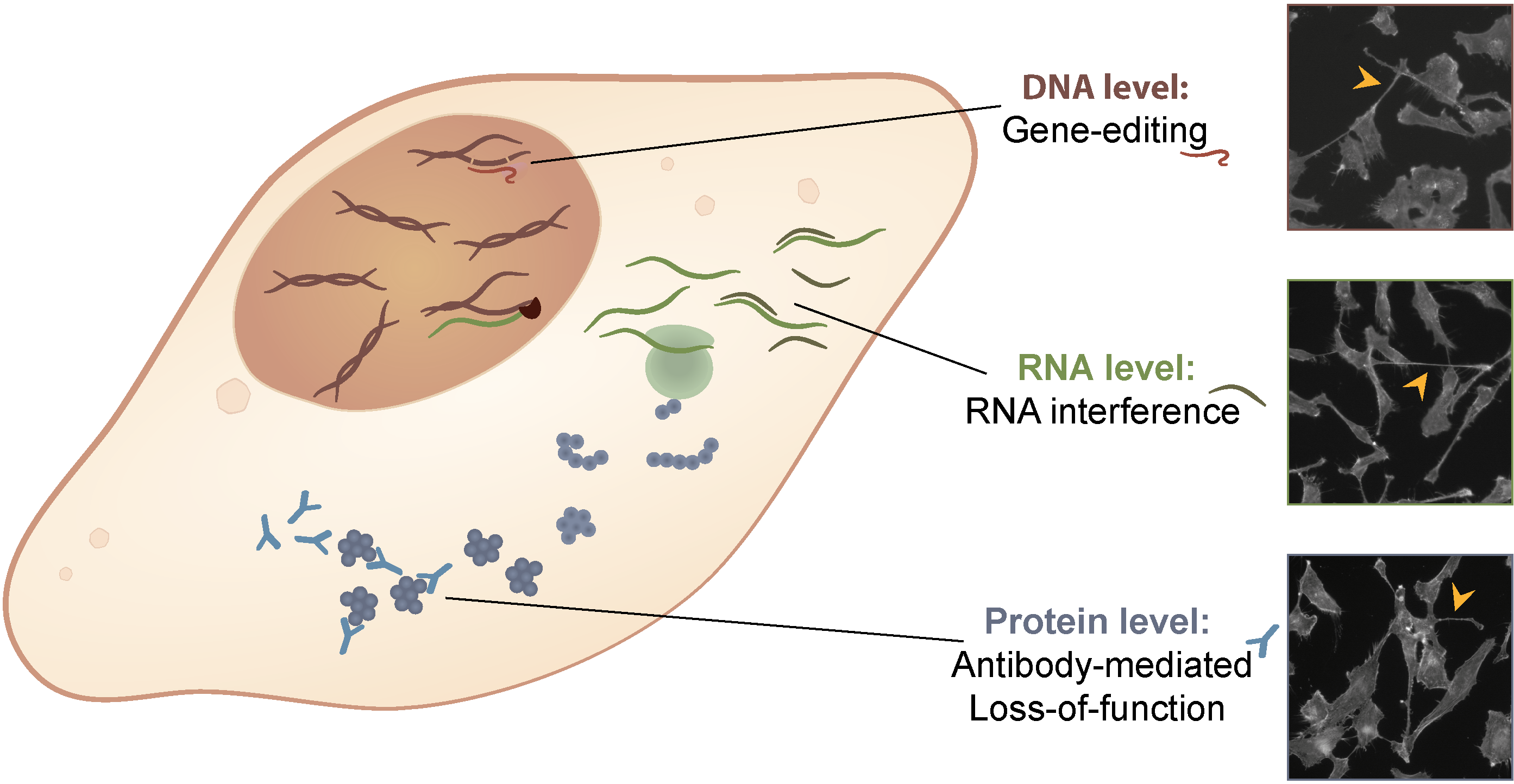
Technology: As part of a collaborative effort (Erfle group), we have developed quantitative cell-based assays and high-content imaging techniques that enable the identification and characterization of numerous novel trafficking regulators. We manipulate cellular functions across all major regulatory levels: at the DNA level via CRISPR-Cas9 gene editing, at the RNA level via RNA interference, and at the protein level via antibody-mediated loss-of-function. The combined use of these three experimental modalities enhances the output of phenotype-based screens and strengthens the validation of their results.
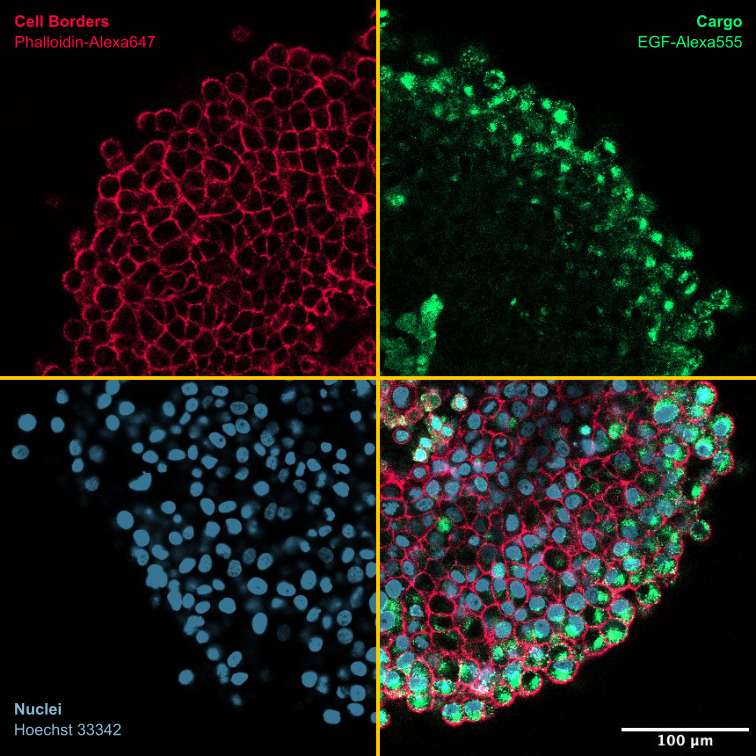
3D models: To achieve greater physiological relevance, we focus on investigating the principles of intracellular trafficking in 3D cell culture systems. In collaboration with data modeling groups (Kummer and Sahle group), we aim to develop predictive models of cargo diffusion, internalization, and endosomal release within these 3D environments. To this end, we study individual cells using high-resolution microscopy and single-cell analysis methods (Rippe group)
Biomedical models: Ultimately, we aim to characterize trafficking processes in disease-relevant contexts. Our current model system involves vascular smooth muscle cells (VSMCs), which respond to numerous intra- and extracellular cues by transitioning between a healthy differentiated (contractile) and an unhealthy dedifferentiated (synthetic) state. We investigate how intracellular trafficking pathways contribute to the regulation of this phenotypic switch, with a focus on identifying specific trafficking events and molecular players that modulate this cellular transition.


Prof. Dr. Vytautė Starkuvienė-Erfle
- +49 (0)6221 54-51259
- vytaute.starkuviene@bioquant.uni-heidelberg.de
- BioQuant, Im Neuenheimer Feld 267, Room 204a
At Vilnius University
- vytaute.starkuviene-erfle@gmc.vu.lt
- Life Science Centre of Vilnius University, Saulėtekio alėja 7, Vilnius, Lithuania, Room 235
Selected Publications
Jian Zhang, Vytaute Starkuviene, Holger Erfle, Zhaohui Wang, Manuel Gunkel, Ziwei Zeng, Carsten Sticht, Kejia Kan, Nuh Rahbari & Michael Keese
Scientific Reports volume 12, Article number: 3498 (2022)
Shijie Liu, Waqar Majeed, Pranas Grigaitis, Matthew J. Betts, Leslie K. Climer, Vytaute Starkuviene & Brian Storrie
Front Cell Dev Bio. 2019
Facile One Step Formation and Screening of Tumor Spheroids Using Droplet-Microarray Platform
Anna A Popova, Tina Tronser, Konstantin Demir, P Haitz, Karolina Kuodyte, Vytaute Starkuviene, Piotr Wajda & Pavel A Levkin
Small.2019 Jun;15(25):e1901299